“It was a dark and stormy decade, and all throughout the world” . . . Wait! That’s not how the story starts!!?? Oh, but yes my dear, it does! In fact, the prequel actually begins in the early 1990’s, a full 28 years before our dark and stormy decade began. Our story would even be promoted and warned about by the likes of Bill Gates and John Hopkins Center for Health! According to Business Insider on April 27, 2018:
“Gates presented a simulation by the Institute for Disease Modeling that found that a new flu like the one that killed 50 million people in the 1918 pandemic would now most likely kill 30 million people within six months.”
We don’t know anything about that over the years spanning 2020 to 2022 now do we?
Insider also claimed:
“According to Gates, a small non-state actor could build an even deadlier form of smallpox in a lab.”
The link they provide is not comforting!
These comments by Business Insider were found in an article on the John Hopkins Center for Health page of media promoting a tabletop exercise for a fictitious global pandemic.
In July of 2018, Business Insider announced the headline: The FDA just approved a drug to treat smallpox in case of a bioterrorism attack — here’s why that scenario is so scary
 In this article, Insider says:
In this article, Insider says:
“The Food and Drug Administration (FDA) announced on July 13 that it had for the first time approved a drug that could treat smallpox if it were ever released as a weapon in a terrorist attack. The medication is called TPOXX (tecovirimat).
“To address the risk of bioterrorism, Congress has taken steps to enable the development and approval of countermeasures to thwart pathogens that could be employed as weapons,” FDA Commissioner Scott Gottlieb said in a statement. “Today’s approval provides an important milestone in these efforts. This new treatment affords us an additional option should smallpox ever be used as a bioweapon.””
This is where we come to Gate’s statements above. Further down the same article, we come to this concerning quote:
“Experts think that if a flu like the 1918 version were to emerge again, it could kill 30 million people within six months. Even scarier flus are possible, too: In ongoing studies in 2014 that were resumed in the past year, scientists demonstrated how the flu virus can be made more deadly. The also showed that deadlier viruses can be engineered to become more contagious.”
 What was COVID-19 regularly likened to over the past 2.5 years??? Why would a flu compared to 1918 and smallpox appear in the same article, back in 2018, two years before our Dark and Stormy Decade?!
What was COVID-19 regularly likened to over the past 2.5 years??? Why would a flu compared to 1918 and smallpox appear in the same article, back in 2018, two years before our Dark and Stormy Decade?!
But now, in the same article, we come to the John Hopkins tabletop roleplay game reference:
“In May, the Johns Hopkins Center for Health Security ran a simulation demonstrating what might happen if a fringe group were to release a modified disease related to the Nipah virus. An outbreak of the little-known Nipah virus in India in May sickened at least 18 and killed 17 of those infected.
In the Johns Hopkins simulation, the modified virus killed more than 150 million people within a year.”
This so-called, “little-known” virus is the focus of a tabletop simulation two years before 2020. Now, I told you the prequel to our “Dark and Stormy Decade” began 28 years earlier, NOT back in 2018! Our prequel has introduced you to a very wealthy man, and a University/Research center. I will now introduce you to a third character in our prequel. Way back in 1994, we have the following story as reported by Christopher C. Broder Ph.D. & Kum Thong Wong Ph.D. in their 2016 article: Henipaviruses. Remember Christopher’s name!
“A new paramyxovirus was isolated and identified in 1994 in an outbreak of fatal cases of respiratory disease in horses and humans in the Brisbane suburb of Hendra, Australia, and was shown to be distantly related to measles virus and other morbilliviruses (Murray et al. 1995a).
. . . Near Mackay in central Queensland, ~1000 km north of Brisbane, a farmer experienced a brief aseptic meningitic illness after caring for and assisting at the necropsies of two horses that were only later shown to have died from this virus infection (Hooper et al. 1996; Rogers et al. 1996). Thirteen months later this individual suffered severe fatal encephalitis resulting from that initial virus infection characterized by uncontrolled focal and generalized epileptic activity (O’Sullivan et al. 1997). The virus was provisionally termed equine morbillivirus but was later re-named HeV where the initial recognized outbreak had occurred.”
HeV is short for Hendra Virus, the He in Henipavirus. Let’s take a closer look at this “new” virus.
What is Henipavirus?
Last Updated: Jun 28, 2019
“HeV infection in horses, and then in humans, was first reported in 1994 in Australia. In contrast, NiV infection was first observed in pigs and subsequently in humans in 1998, in Malaysia.
“The disease manifests in 4-20 days or 5-12 days, for HeV and NiV infection respectively. It presents as fever with acute encephalitis, or as an acute influenza-like illness leading to severe respiratory illness, or as meningitis.
“Why are henipaviruses so deadly? The answer lies in their ability to encode several proteins which block the innate immune response in infected animals and humans. These inhibit the cell’s response to viral infection, and allow viral replication. These thus act as virulence factors, blocking the interferon-stimulated antiviral defense mechanisms from kicking in inside the infected cells. The virus causes destruction of small blood vessels in many major organs, such as the brain, liver and kidney, causing organ failure. This is associated with microinfarction, infection, and organ failure.”
Notice the year of the last article quoted above? Mark that in your memory as we move along in our prequel. Dates and names are important! Now that we know a little about this “little-known” virus, we can scoot back to an earlier year of some importance to our timeline.
Let’s look at two quotes from 2013, a year that carries yet more interest.
The changing face of the henipaviruses
Emma L.Croserm Glenn A.Marsh
CSIRO Animal, Food and Health Sciences, Australian Animal Health Laboratory, Private Bag 24, Geelong 3220, Australia
“Over the past several years, we have seen an increase in the number of cases and an altered clinical presentation of Hendra virus in naturally infected horses. Recent increase in the number of cases has also been reported with human Nipah virus infections in Bangladesh. These factors, along with the recent discovery of henipa and henipa-like viruses in Africa, Asia and South and Central America adds, a truly global perspective to this group of emerging viruses.”
Received 11 April 2013, Revised 12 July 2013, Accepted 5 August 2013, Available online 13 August 2013.
First, we learn of the spread of Henipavirus above. Then below, we discover a 2012 paper released for public viewing in 2013! This is important information. Where have we heard about the necessity of boosting our Interferons before?
Type I Interferon Signaling Protects Mice From Lethal Henipavirus Infection
Journal of Infectious Diseases Advance Access published November 16, 2012
Journal of Infectious Diseases, 2013
“IFN-I can exert antiviral effects at multiple levels, causing the induction of antiviral genes as well as augmenta-
tion of antigen-presenting cell and lymphocyte functions.”“It has been hypothesized that the ability of IFNs to inhibit cell death may preserve neuronal populations and limit disease in the central nervous system (CNS) induced by either viral infection or inflammation [29].”
“The selective increased permissiveness of primary brain glial cultures lacking IFNAR signaling after infection with NiV at low but not high MOI strongly suggests that mouse cells can efficiently detect NiV infection and secrete IFN-I to protect surrounding cells from virus spreading, although NiV-induced production of IFN-I by neuronal cells may be limited [30]. Only a small population of infected neurons produces IFN-I following infection with certain viruses, and IFN-I was shown to be principally made by parenchymal cells in the brain [31]. Our results have demonstrated the critical importance of IFNAR signaling for the protection of mice from henipavirus infection in vivo.”
“Altogether, these results are consistent with infection of IFNAR-KO mice with other neurotropic RNA viruses, including vesicular stomatitis virus [20], measles [33], coronavirus [34], or West Nile virus infection [35], in which the absence of IFNAR downstream signaling highly increases the susceptibility to infection.”
“Furthermore, the critical role of IFN-I signaling in control of the HeV and NiV infection observed in this study, and recent observations that in human cell lines NiV and HeV inhibit IFN-I production rather than IFN-I signaling pathways [37], as well as previous finding that interferon inducer poly(I)-poly(C(12)U) could prevent Nipah virus-induced mortality [38], suggest a potential for IFN-I treatment as a possible postexposure therapeutic.”
Even more interesting is the reference above, regarding how Type 1 Interferons protected the mice from contracting encephalitis and the study on West Nile.
In 2014. we read of an event that took place in China in 2012 as well. Hmmm, perhaps 2012 was a more pivotal year in this timeline than previously noted:
Volume 20, Number 6—June 2014
Letter
Novel Henipa-like Virus, Mojiang Paramyxovirus, in Rats, China, 2012
“In June 2012, in Mojiang Hani Autonomous County, Yunnan Province, China, severe pneumonia without a known cause was diagnosed in 3 persons who had been working in an abandoned mine; all 3 patients died. MojV shares similar features with known henipaviruses.
“Of 9 anal swab samples from the R. flavipectus rats, 3 were positive for MojV, and a tissue sample from 1 of the 3 MojV-positive rats was also MojV positive (tissue was not collected from the other 2 rats).
“Our study showed the presence of a rodent-origin, henipa-like virus, MojV, in China. R. flavipectus rats are the natural reservoir of MojV. This finding and its context indicate that Henipavirus spp. viruses might infect more mammalian hosts than previously thought and that bats may not be the only hosts of henipaviruses.”
After 2014, our prequel’s plot begins to form. Keep in mind the above quote from a letter in 2014 as you read the following:
 Henipaviruses
Henipaviruses
Christopher C. Broder Ph.D. & Kum Thong Wong Ph.D.
Chapter
First Online: 09 September 2016
“The recently identified third henipavirus isolate, Cedar virus (CedPV), is not pathogenic in animals susceptible to HeV and NiV disease. Molecular detection of additional henipavirus species has been reported but no additional isolates of virus have been reported. Studies have shown that CedPV is not pathogenic in animals susceptible to HeV and NiV disease, nor is it known to be zoonotic.
“The recognized natural reservoir hosts of HeV, NiV, and CedPV are pteropid bats, which do not show clinical illness when infected.
“…to date HeV, NiV, and CedPV are the only virus isolates that have been reported (Wu et al. 2014; Drexler et al. 2012).
“Most recently, a novel henipa-like virus, Mojiang paramyxovirus (MojV), was identified in rats (Rattus flavipectus) in China by nucleic acid analysis, with a genome length of 18,404 nt; however no virus isolate was obtained (Wu et al. 2014).”
Did you catch that? No virus isolate was obtained! There is another prop in our story to take note of. References to bats as the primary source of viral infestation. We haven’t heard that used before, now. . . have we? Hmmm.
This 2016 article introduces some alarming facts and figures about the lethality of this family of viruses, but goes on to discuss treatments and preventions from a mainstream medical viewpoint. The article itself is quite long, so as lengthy as these quotes are, they are far from the entire thing! Click the article title to read the entire article at your leisure. Lets look at some of these quotes from this article:
“…nearly annual outbreaks of NiV infection have now been recognized since 2001, occurring primarily in Bangladesh but also India. The most recent cases of human infections occurred in early 2015 with two fatalities (Anonymous 2015). The spillovers of NiV in Bangladesh and India have had lower numbers of human infections; however the fatality rates have been notably higher from 75 to 100 %.
“Both HeV and NiV are highly pathogenic in a number of mammalian species and possess several characteristics that distinguish them from all other known paramyxoviruses and are classified as Biosafety Level-4 (BSL-4) agents.”
Note that biosafety level. Remember the notes earlier in our prequel about bioweapons? Hmmm. Carrying on here:
(Nipah Virus) “The risk factors for severe disease and poor prognosis included abnormal doll’s eye reflex, tachycardia, and the presence of virus in the cerebrospinal fluid (Chua et al. 2000b), and diabetes mellitus (Chong et al. 2001b).
“NiV infection could also take a chronic and quiescent course with neurological disease occurring later (>10 weeks) following a non-encephalitic or asymptomatic infection. A recrudescence of neurological disease, also termed relapsing encephalitis, was also observed in some patients who had previously recovered from an acute encephalitic infection. Here, there is a recrudescence of virus replication in the CNS. Most reported cases of relapsed encephalitis presented from a few months to approximately 2 years following the initial acute infection, however two cases of relapsed encephalitis were observed in 2003 4 years later (Wong et al. 2001; Chong and Tan 2003; Tan and Wong 2003) and the longest reported case of NiV encephalitic recrudescence is 11 years (Abdullah et al. 2012).”
“There is no evidence of HeV shedding in people who have recovered from infection (Taylor et al. 2012).”
So a) we have a virus whose payload can be anywhere from immediate all the way out to 11+years before a person has seen the last of it. Not comforting to say the least. This article’s authors now go on to discuss potential treatments and preventions:
“Chloroquine, an anti-malarial drug, was shown to block the critical proteolytic processing needed for the maturation and function of the HeV F glycoprotein discussed earlier (Pager et al. 2004) and could block infection in cell culture (Porotto et al. 2009). However, chloroquine and ribavirin treatment of a HeV-infected individual had no clinical benefit (reviewed in Broder et al. 2013). . . with HeV challenge monkeys treated with ribavirin having marked increases of neurological symptoms. C”
Are they just saying this to be in mainstream medical’s good books, or did Chloroquine actually have no effect on human patients? (Hint: pay attention to a COI statement later in this round of quotes) We won’t forget the unnecessary shootdown of Hydroxychloroquine during the covid pandemic! HCQ very much assisted with fighting off covid, as can the entire group of quinones.
“Also, various forms of poly(I:C) are strong inducers of IFN-α and -β production, have been explored as antiviral therapies for over 40 years. PolyIC12U is very specific in triggering the Toll-like receptor (TLR)3 pathway (reviewed in Nicodemus and Berek 2010). PolyIC12U was shown capable of blocking NiV replication, and continuous administration of polyIC12U for 10 days beginning at the time of challenge was shown to prevent lethal NiV disease in five of six hamsters (Georges-Courbot et al. 2006), suggesting that use of TLR3 agonists such as PolyIC12U, perhaps in combination with other antiviral strategies, should be explored.”
Remember the mice earlier? Remember the advice about keeping your Interferons boosted?
But now our prequel introduces us to another event in our current Dark and Stormy Decade as we continue to quote from the same article. Que the Insider article quoted earlier where smallpox had been discussed.
“Another poxvirus-based vaccine was examined as a potential livestock vaccine using recombinant canarypox virus in pigs (Weingartl et al. 2006). Here, the NiV F and G glycoprotein genes were used to generate recombinant canarypox viruses (ALVAC) vaccine vectors and used to immunize pigs. ALVAC vectors expressing F and G were tested alone and in combination, and piglets were challenged intranasally with NiV. Here, protection from NiV-mediated disease was seen in all vaccinated pigs by either ALVAC vector alone or in combination and that vaccinated animals shed only low levels of nucleic acid detectable virus with no isolatable virus (Weingartl et al. 2006).”
So we had no shedding from Type 1 Interferon boosting, but we do get low levels of viral shedding from this livestock vaccine based on a poxvirus. Remember this!
“More recently, several viral vector-based henipavirus vaccines have also been examined in animal challenge studies; these have included immunizations using the vesicular stomatitis virus based platform (VSV) expressing either the NiV G or F glycoprotein in the hamster model (DeBuysscher et al. 2014; Lo et al. 2014) and also VSV-based vaccines using NiV F or G in the ferret model (Mire et al. 2013). All these studies demonstrated that a single dose of vaccine could induced strong neutralizing antibody responses and could afford protection from NiV challenge, highlighting their potential usefulness as either a livestock vaccine or one suitable in an emergency use or outbreak scenario . Vaccination and challenge experiments have also been examined using an adeno-associated virus platform with NiV G showing protection against challenge in the hamster model and low level cross-protection (three of six animals) against a HeV challenge (Ploquin et al. 2013), and also a recombinant measles virus vector with NiV G which showed two of two AGMs were protected from NiV challenge (Yoneda et al. 2013).”
 HOLD THE PHONE! Christopher and Kum cite a 2013 reference and two 2014 references in relation to a viral vector we now know was used in creating the covid “vaxx”! (If you have not read my own article on this, please click the link to review.) We even have Big Pharma companies using adenoviruses in their so-called “vaxx” formulations, but with very similar side effects to those using the VSV vector. Ok, carrying on here:
HOLD THE PHONE! Christopher and Kum cite a 2013 reference and two 2014 references in relation to a viral vector we now know was used in creating the covid “vaxx”! (If you have not read my own article on this, please click the link to review.) We even have Big Pharma companies using adenoviruses in their so-called “vaxx” formulations, but with very similar side effects to those using the VSV vector. Ok, carrying on here:
“The simplicity and inherent safety of the HeV-sG subunit vaccine approach together with the numerous successful vaccination and challenge studies that have been carried out in multiple animal models, the HeV-sG subunit vaccine was chosen for the development of an equine vaccine to prevent infection in horses and also reduce the risk of HeV transmission to people. HeV-sG was licensed by Zoetis, Inc. (formerly Pfizer Animal Health) and developed as an equine vaccine for use in Australia.
The horse vaccine against HeV (Equivac® HeV) is the first commercially deployed vaccine developed against a BSL-4 agent and is the only licensed treatment for henipavirus infection. To date, more than 430,000 doses of Equivac® HeV vaccine have been administered to horses (Zoetis, Inc.).”
Well, well, well, if we don’t get another player in our prequel that we are all too familiar with now in our Dark and Stormy Decade! Why, it’s none other than Pfizer whose former Animal Health division would become Zoetis. Don’t burst a brain-bubble now, but what do you know Pfizer for today?! But wait. . . our prequel continues with another quote from Christopher’s article:
“In 2010, the m102.4 mAb producing cell line was provided to the Queensland Government, Queensland Health, Australia to produce the m102.4 mAb for emergency use on a compassionate basis in future cases of high-risk human HeV exposure. Queensland Health Authorities have completed in May, 2016, the first phase 1 clinical safety trial of m102.4 in human subjects (Queensland 2013). To date, 11 individuals exposed to either HeV in Australia (10 people) or NiV in the United States (1 person) have been given high-dose m102.4 therapy under emergency use protocols, and all have remained well with no associated adverse events. In addition, the vaccine against HeV (Equivac® HeV) is vaccine for horses that is also expected to provide a substantial health benefit to humans, and has fit well within the spirit of a “One Health” approach for the human and animal interface and also in respect to environmental health.”
“Compassionate basis”!!! Provided cell lines in 2010, with the first phase 1 trial completed in 2016 that began in 2013. The real kicker to end off this particular set of quotes, is the reference to Equivac HeV. What other horse medication has successfully been used for humans long before it became a horse treatment? This same treatment would also prove very successful in treating covid-19 and as an effective preventive for that virus as well, but was strongly maligned in a coordinated MSM attack at all levels of public discourse. Hmmm.
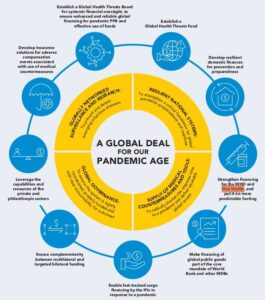
But the last line from the quotes above should be of particular concern going forward, because while many of us only just began hearing about “One Health” this past year or so as being one of WEF’s initiatives, it’s clearly been around for much longer than the pandemic!
Bringing you more on “One Health”, is this next set of quotes from another, more recent article from 2018:
Enhancing preparation for large Nipah outbreaks beyond Bangladesh: Preventing a tragedy like Ebola in West Africa
Halsie Donaldson, Daniel Lucey, in International Journal of Infectious Diseases, 2018
“Nipah is a One Health zoonotic infectious disease linked to fruit bats, and sometimes pigs or horses. We advocate anticipating and preparing for urban and larger rural outbreaks of Nipah. Immediate enhanced preparations would include standardized guidance on infection prevention and control, and personal protective equipment, from the World Health Organization (WHO) on their OpenWHO website and 2018 “Managing Epidemics” handbook, along with adding best clinical practices by experts in countries with multiple outbreaks such as Bangladesh and India. Longer-term enhanced preparations include accelerating development of field diagnostics, antiviral drugs, immune-based therapies, and vaccines. WHO-coordinated multi-partner protocols to test investigational treatments, diagnostics, and vaccines are needed,
“Separate from any naturally-occurring Nipah virus outbreaks, intentional release of Nipah virus has also been recognized as a potential risk. The initial October 2015 publication of the US “Bipartisan Report of the Blue Ribbon Study Panel on Biodefense” included a brief scenario of a hypothetical attack in Washington, DC, and in other locations in the USA and abroad that was due to a ‘genetically modified’ and aerosolized Nipah virus. The zoonotic or ‘One Health’ nature of human infections with Nipah virus was recognized in this scenario, with animal deaths occurring as well as human fatalities. Notably, of the 34 recommendations from this Blue Ribbon panel, recommendation number 7 was to “integrate animal health and One Health approaches into biodefense strategies” (Lieberman and Ridge, 2015). Similarly, on May 15, 2018 an all-day large simulation exercise called ‘Clade X’ was carried out and posted online (Johns Hopkins Center for Health Security). The hypothetical pathogen was a bioengineered transmissible hybrid virus with genes from Nipah virus and human parainfluenza virus. Intentional attacks began in Caracas, Venezuela and in Frankfurt, Germany and then spread to over 300 cities via international travel.
 “Enhancement of preparedness measures for future urban Nipah outbreaks should include heightened surveillance, coordinated rapid diagnostic testing, training exercises including the use of and sufficient supplies of personal protective equipment (PPE), detailed isolation and quarantine protocols, clinical management protocols, and discussion of research protocols and their ethics for use of investigative therapies and vaccines.”
“Enhancement of preparedness measures for future urban Nipah outbreaks should include heightened surveillance, coordinated rapid diagnostic testing, training exercises including the use of and sufficient supplies of personal protective equipment (PPE), detailed isolation and quarantine protocols, clinical management protocols, and discussion of research protocols and their ethics for use of investigative therapies and vaccines.”
Notice the references to bioweapons and attacks using such weapons against nation-states. By the way, we’re back in 2018 now, where I began talking about the prequel to our Dark and Stormy Decade. Back in 2018, they were talking about the need for increased surveillance, rapid testing, personal protective equipment, quarantine protocols, etc.
The bioweapon status of this virus family carries right along in 2019:
Case fatality rate and risk factors for Nipah virus encephalitis: A systematic review and meta-analysis
Sebastien Kenmoe, … Richard Njouom, in Journal of Clinical Virology, 2019
“In addition, the NiV is recognized as a class C biological weapon that includes emerging pathogens that can be used in the context of bioterrorism [4].”
“Findings from this study suggest that NiV Encephalitis is associated with a high CFR and that male gender, travel outside their sub-district, climbing trees, and exposure to pigs and DPS are associated with an increased risk of NiV encephalitis.”
I’m not entirely sure what gender has to do with fatality with the Henipavirus family, but this particular issue may remind you of another pox that the WHO has been trying to whip up concern for during our Dark and Stormy Decade!
Now we come to our Dark and Stormy Decade, which began in March 2020 (technically fall 2019 over in China) with the creation and release of COVID-19. 2.5yrs later, in 2022, another virus emerges in China, this time known as Langya, a supposedly new variant of the Henipavirus! Let us take a moment to look at what is known of this “little-known” virus:
Langya Henipavirus: What Is This New Zoonotic Virus Outbreak That Has Infected 35 People in China So Far?
The Langya henipavirus has been found in China’s Shandong and Henan provinces and can be transmitted from animals to humans, reported Taipei Times.
Published: August 9, 2022 8:57 PM IST
By India.com News Desk
“Effects and transmission of Langya Henipavirus
According to the Taipei Times, the virus can cause renal and hepatic failure and most likely is transmitted from animals to humans. Chuang said the 35 patients in China did not have close contact with each other or a common exposure history, and contact tracing showed no viral transmission among close contacts and family, suggesting that human infections might be sporadic.
Signs and symptoms of Langya Henipavirus
The 26 patients developed symptoms including fever, fatigue, a cough, loss of appetite, muscle pain, nausea, headache and vomiting. They also showed a decrease in white blood cells, low platelet count, liver failure and kidney failure.”
This sounds very familiar to symptoms already out there for Henipa. In addition, the above info is being parroted around the world as of August 9th 2022, almost verbatum across all sources. I want to back up a moment to 2019 again as we look at how this virus affects the body:
2019 Mar 1;77(2):ftz023. doi: 10.1093/femspd/ftz023.
Henipavirus infection of the central nervous system
Brian E Dawes 1 2, Alexander N Freiberg 2 3 4
Affiliations expand
PMID: 30985897 PMCID: PMC6974701 DOI: 10.1093/femspd/ftz023
“While outbreaks of Nipah and Hendra virus infections remain rare and sporadic, there is concern that NiV has pandemic potential. Despite increased attention, little is understood about the neuropathogenesis of henipavirus infection. Neuropathogenesis appears to arise from dual mechanisms of vascular disease and direct parenchymal brain infection, but the relative contributions remain unknown while respiratory disease arises from vasculitis and respiratory epithelial cell infection.”
Viral hemorrhagic fever: Molecular pathogenesis and current trends of disease management-an update
Received 17 April 2021, Revised 2 July 2021, Accepted 4 July 2021, Available online 8 July 2021, Version of Record 16 July 2021.
“It is believed that the severe symptoms manifested in VHF (viral hemorrhagic fever) are largely due to the host immune responses to the virus rather than virus-induced cytopathology.”
You were paying attention to the notes about Type 1 Interferon behaviour earlier, correct?
“Nipah and Hendra viruses cause encephalitis, although respiratory and influenza-like presentations are also common. Diagnosis is confirmed with serology or molecular testing.”
“Nipah and henipaviral diseases have been added to the World Health Organization’s R&D Blueprint list of epidemic threats that require urgent research and development action.[4]” (foot note led to “This page cannot be found” on the WHO website.”
Last reviewed: 9 Jul 2022
Last updated: 15 Oct 2021
But remember, we have a vaccine!!! (The rallying cry for The Dark and Stormy Decade) The following quotes have dates and claims that make the August 9th worldwide announcement seem a little out of date! See if you can spot them:
Henipavirus glycoprotein architecture suggests therapeutic strategies
Bioengineer BY BIOENGINEER March 4, 2022 in Cancer
“There is a vaccine approved for use in horses and a modified version entered a human clinical trial.
New attempts to design life-saving preventatives and treatments became even more urgent after a new strain of Hendra was discovered a few months ago.
“They also observed that a mixture or “cocktail” of antibodies work better together to disarm Nipah viruses. Similar synergistic effects were seen in a set of antibodies against Hendra viruses. This combining of forces also helped keep escape mutants from emerging to sidestep the antibody response.
“The paramyxovirus is a large family of single-strand RNA viruses. They cause several distinct types of diseases, most of which are transmitted on respiratory droplets. They include measles, mumps, distemper, parainfluenza, and the henipavirus diseases that have more recently crossed from animals to humans.
“These findings, the researchers noted, “provide a blueprint for engineering next-generation vaccine candidates with improved stability and immunogenicity.” The s would focus on the vulnerability of the head domain. They anticipate a design approach like that employed for newer computer-engineered SARS-CoV-2 and respiratory syncytial virus candidates. (emphasis mine) A mosaic of head antigens would be presented to the body in an ordered array on a multivalent display. Using only the head domain rather than the full G protein could also make manufacturing large supplies of vaccine simpler.
“COI Statement
“Competing interests: ChristopherC. Broder .. is a United States federal employee and co-inventor on US and foreign patents pertaining to soluble forms of Nipah virus and Hendra virus G glycoproteins and human monoclonal antibodies against Hendra and Nipah viruses; Broder and Moushimi Amaya are co-inventors on US and foreign patents pertaining to Cedar virus and methods of use and recombinant Cedar virus chimeras, whose assignees are the US as represented by the Henry M. Jackson Foundation for the Advancement of Military Medicine (Bethesda, MD, USA).”
The quote above was talking about monoclonal antibodies, but there are bigger claims in the above quotes from that article! “Computer-engineered SARS-CoV-2”. . . Computer-engineered!!! Who said this?! The article’s amazingly-included Conflict of Interest paragraph drops several nuclear bombs! For starters, two wonderfully-helpful articles that I’d quoted from so extensively earlier in our prequel, were written by a US Federal Employee and co-inventor on US and foreign patents pertaining to soluble forms of Henipa G glycoproteins and monoclonial antibodies against those viruses!!! He’s also working on an Henipa chimera from the Cedar variant whose assignees are represented by military medicine!!! Hoo boy! It’s pretty safe to say, that on the Henipavirus plot line, Christopher C. Broder is a key player and no doubt, one of the string pullers behind mainstream medical behaviour, from a Big Pharma governmental position.
This article touches on some of the things that can be done to prevent or deal with this viral “newcomer”. Next up, is a blog article on natural ways and means to deal with Henipavirus.